In this May 2025 edition of Editors’ Picks, we spotlight standout studies selected by the editors of the 10 peer-reviewed journals published by the American Association for Cancer Research (AACR). This month’s selections include research revealing genetic parallels between tumor adaptation to hypoxia and high-altitude physiology, innovative strategies to reprogram the tumor microenvironment, and new evidence linking oil and gas exposure to childhood leukemia risk. Together, these studies reflect dynamic progress in cancer prevention, diagnosis, treatment, and survivorship.
Follow the links for the full text of each article, which are freely available for a limited time.
Journal: Blood Cancer Discovery
Immune Escape of Acute Myeloid Leukemia after Transplantation
Acute myeloid leukemia (AML) is the main indication for allogeneic hematopoietic cell transplantation, but relapse is common because of chemotherapy resistance and immune escape. We discuss the mechanisms of AML immune evasion including loss or downregulation of MHC class I and II, reduced tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor expression, inhibitory metabolite production, inhibitory ligand expression, impaired proinflammatory cytokine production, and AML niche alterations. Understanding and targeting these mechanisms could enhance outcomes for patients with AML undergoing allogeneic hematopoietic cell transplantation and immunotherapies.
Significance: We discuss the mechanisms of AML immune evasion including loss or downregulation of MHC class I and II, reduced TRAIL receptor expression, inhibitory metabolite production, inhibitory ligand expression, impaired proinflammatory cytokine production, and AML niche alterations.
Journal: Cancer Discovery
Convergent Genetic Adaptation in Human Tumors Developed Under Systemic Hypoxia and in Populations Living at High Altitudes
This study explores parallels between systemic hypoxia adaptation in high-altitude populations and tumorigenesis. We identified EPAS1, a gene critical for hypoxia adaptation in populations such as Tibetans and Sherpas, as playing a similar adaptive role in tumors arising under hypoxic conditions. Tumors from patients with chronic hypoxia displayed impaired DNA repair and frequent emergence of EPAS1 variants, with frequencies reaching up to 90%, echoing the positive selection seen in high-altitude dwellers. Mechanistically, EPAS1 gain-of-function mutations promote COX4I2 expression, reducing cellular oxygen consumption and supporting tumor proliferation in hypoxia. Analysis of clinical data from patients with hypoxia revealed tissue-specific and time-sensitive tumorigenic effects, particularly impacting oxygen-sensitive cells in the postnatal period. Our findings suggest that EPAS1-driven adaptation mechanisms in high-altitude populations provide a model for understanding tumor evolution under hypoxic stress, highlighting how genetic adaptations to diverse stressors in natural populations may yield insights into tumorigenesis and cancer progression.
Significance: This study reveals a broad convergence in genetic adaptation to hypoxia between natural populations and tumors, suggesting that insights from natural populations could enhance our understanding of cancer biology and identify novel therapeutic targets.
A related commentary was published in the May issue, and the study was also featured on the issue’s cover.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Exposures from Oil and Gas Development and Childhood Leukemia Risk in Colorado: A Population-Based Case–Control Study
Background: Children living in upstream oil and natural gas (O&G) areas may be exposed to leukemogens and at increased risk for acute lymphoblastic leukemia (ALL).
Methods: We conducted a case–control study of children born in Colorado between 1992 and 2019. We matched 451 children diagnosed with ALL at ages 2 to 9 years starting in 2002 to 2,706 controls based on birth month/year and Hispanic ethnicity. We estimated upstream O&G activity intensities from conception through a 10-year latency using our intensity-adjusted inverse distance weighted (IA-IDW) model. We applied logistic regression models adjusted for confounders to evaluate associations between ALL and IA-IDW.
Results: For children within 5 km of an O&G well site, we observed a 62% [OR = 1.62; 95% confidence interval (CI), 0.964–2.62], 84% (OR = 1.84; 95% CI, 1.35–2.48), and 100% (OR = 2.00; 95% CI, 1.14–3.37) increase in ALL risk for low, medium, and high IA-IDW groups, compared with the referent group. Within 13 km, we observed a 59% (OR = 1.59; 95% CI, 1.03–2.37), 40% (OR = 1.40; 95% CI, 1.09–1.80), and 164% (OR = 2.64; 95% CI, 1.80–3.86) increase in ALL risk for low, medium, and high IA-IDW groups.
Conclusions: Colorado’s children living within 13 km of O&G well sites are at increased risk for ALL, with children within 5 km bearing the greatest risk. Current setbacks between O&G well sites and residences may not be sufficient to protect the health of these children.
Impact: Our results can be applied to policies to reduce childhood leukemogen exposures.
This article was highlighted in the May issue.
Journal: Cancer Immunology Research
Selective STING Activation in Intratumoral Myeloid Cells via CCR2-Directed Antibody–Drug Conjugate TAK-500
The tumor microenvironment in solid tumors contains myeloid cells that modulate local immune activity. Stimulator of IFN gene (STING) signaling activation in these myeloid cells enhances local type-I IFN production, inducing an innate immune response that mobilizes adaptive immunity and reprograms immunosuppressive myeloid populations to drive antitumor immunity. In this study, we generated TAK-500, an immune cell–directed antibody–drug conjugate, to deliver a STING agonist to CCR2+ human cells and drive enhanced antitumor activity relative to nontargeted STING agonists. Preclinically, TAK-500 triggered dose-dependent innate immune activation in vitro. In addition, a murine TAK-500 immune cell–directed antibody–drug conjugate surrogate enhanced innate and adaptive immune responses both in in vitro and murine tumor models. Spatially resolved analysis of CCR2 and immune cell markers in the tumor microenvironment of >1,000 primary human tumors showed that the CCR2 protein was predominantly expressed in intratumoral myeloid cells. Collectively, these data highlight the clinical potential of delivering a STING agonist to CCR2+ cells.
This article was featured on the cover of the May issue.
Journal: Cancer Prevention Research
Chronic Cigarette Smoke Exposure Masks Pathological Features of Helicobacter pylori Infection While Promoting Tumor Initiation
Gastric cancer is the fifth most common cancer and the fifth leading cause of cancer deaths worldwide. Chronic infection by the bacterium Helicobacter pylori is the most prominent gastric cancer risk factor, but only 1% to 3% of infected individuals will develop gastric cancer. Cigarette smoking is another independent gastric cancer risk factor, and H. pylori–infected smokers are at a 2- to 11-fold increased risk of gastric cancer development, but the direct impacts of cigarette smoke (CS) on H. pylori pathogenesis remain unknown. In this study, male C57BL/6 mice were infected with H. pylori and began smoking within 1 week of infection. The mice were exposed to CS 5 days/week for 8 weeks. CS exposure had no notable impact on gross gastric morphology or inflammatory status compared with filtered-air (FA) exposed controls in mock-infected mice. However, CS exposure significantly blunted H. pylori–induced gastric inflammatory responses, reducing gastric atrophy and pyloric metaplasia development. Despite blunting these classic pathological features of H. pylori infection, CS exposures increased DNA damage within the gastric epithelial cells and accelerated H. pylori–induced dysplasia onset in the INS-GAS gastric cancer model. These data suggest that cigarette smoking may clinically silence classic clinical symptoms of H. pylori infection but enhance the accumulation of mutations and accelerate gastric cancer initiation.
Prevention Relevance: These findings suggest that cigarette smoking suppresses pathophysiological hallmarks of H. pylori infection while accelerating gastric carcinogenesis. Therefore, smokers should receive screening for H. pylori infection to reduce gastric cancer risk.
A related commentary was published in the May issue, and the study was also featured on the issue’s cover.
Journal: Cancer Research (May 1 Issue)
GLI2 Facilitates Tumor Immune Evasion and Immunotherapeutic Resistance by Coordinating Wnt Ligand and Prostaglandin Signaling
Therapeutic resistance to immune checkpoint blockade has been commonly linked to the process of mesenchymal transformation (MT) and remains a prevalent obstacle across many cancer types. An improved mechanistic understanding for MT-mediated immune evasion promises to lead to more effective combination therapeutic regimens. Herein, we identified the hedgehog transcription factor, GLI2, as a key node of tumor-mediated immune evasion and immunotherapy resistance during MT. GLI2 generated an immunotolerant tumor microenvironment through the upregulation of WNT ligand production and increased prostaglandin synthesis. This pathway drove the recruitment, viability, and function of granulocytic myeloid-derived suppressor cells while also impairing type I conventional dendritic cell, CD8+ T-cell, and NK cell functionality. Pharmacologic inhibition of EP2/EP4 prostaglandin receptor signaling or WNT ligand secretion each reversed a subset of the immunomodulatory effects of GLI2 and prevented primary and adaptive resistance to anti–PD-1 immunotherapy, respectively. A transcriptional GLI2 signature correlated with resistance to anti–PD-1 immunotherapy in patients with stage IV melanoma. Together, these findings provide a translational roadmap to direct combination immunotherapies in the clinic.
Significance: WNT and prostaglandin signaling generate an immunotolerant environment in GLI2-active tumors and can be targeted as a component of immunotherapeutic combination strategies to overcome resistance in tumors exhibiting mesenchymal plasticity.
Journal: Cancer Research (May 15 Issue)
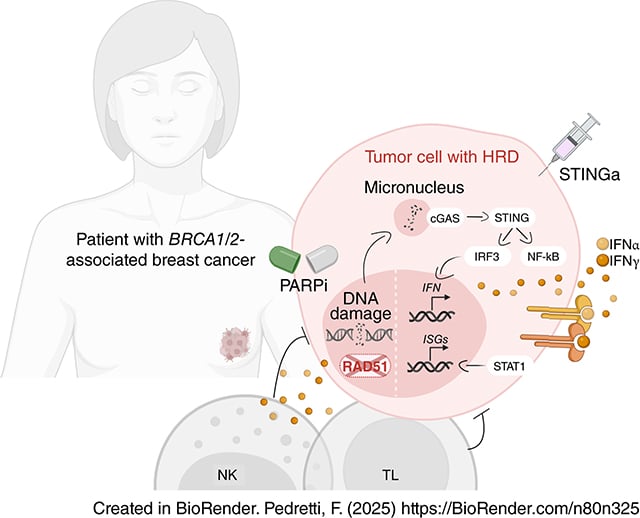
Harnessing STING Signaling and Natural Killer Cells Overcomes PARP Inhibitor Resistance in Homologous Recombination–Deficient Breast Cancer
Homologous recombination deficiency (HRD) contributes to genomic instability and leads to sensitivity to PARP inhibitors (PARPi). HRD also activates the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING)–interferon pathway, highlighting the need to understand the impact of cGAS–STING–IFN signaling on PARPi efficacy. In this study, we analyzed a cohort of 35 breast cancer patient-derived xenografts and mouse-derived allografts. PARPi sensitivity correlated with HRD, increased genomic instability, and activation of the cGAS–STING–IFN signaling pathway. Single-cell analyses showed that IFN signaling and IFN-based immune interactions were suppressed in preclinical models with acquired resistance to PARPi, lacking concomitant clonal expansion of functional CD8+ T cells. However, the combination of a PARPi and a novel STING agonist (STINGa) increased immune infiltration and resulted in superior antitumor activity in these tumors. Notably, the efficacy of PARPi monotherapy and the combination treatment with a STINGa was dependent on natural killer (NK) cells. In agreement, patients with breast cancer with BRCA1/BRCA2 mutations and good responses to PARPi showed higher abundancy of CD56+ NK cells in the tumor microenvironment and treatment-engaged CD56bright NK cells in the peripheral immune compartment, compared with those with poor responses. Therefore, these findings propose the combination of PARPis and a STINGa as a potential novel strategy to enhance the therapeutic response in patients with acquired PARPi resistance and highlight a pivotal role of NK cells in the PARPi antitumor activity.
Significance: PARP inhibitor sensitivity is associated with cGAS–STING–IFN signaling, which can be harnessed by combining PARP inhibitors with STING agonists to overcome acquired resistance and requires NK cells to mediate antitumor immunity.
A related commentary was published in the May 15 issue.
Journal: Clinical Cancer Research (May 1 Issue)
Pembrolizumab with Chemotherapy for Patients with Recurrent or Metastatic Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma: A Prospective Phase ll Study
Purpose: Patients with recurrent or metastatic sinonasal squamous cell carcinoma (R/M SNSCC) lack standardized systemic treatment and prospective studies. We evaluated the antitumor response and safety of pembrolizumab with nab-paclitaxel and platinum in R/M SNSCC.
Patients and Methods: Patients with R/M SNSCC received pembrolizumab 200 mg and nab-paclitaxel 260 mg/m2 plus cisplatin 75 mg/m2 or carboplatin at an AUC 5 on day 1 every 21 days for up to six cycles, followed by pembrolizumab maintenance until progression or unacceptable toxicity or 35 cycles. The primary endpoint was the objective response rate (ORR). Secondary endpoints were disease control rate, progression-free survival (PFS), overall survival (OS), and safety. IHC and high-resolution sequencing of the tumor samples were performed.
Results: The ORR in 20 patients was 60.0% (95% confidence interval, 36.1%–80.9%), and two patients (2/20, 10%) achieved complete response. The disease control rate was 100%. The median follow-up was 18.1 months (range, 5.2–31.7), the median PFS was 12.2 months (95% confidence interval, 9.0 months–not estimated), and the median OS was not reached. Patients with a PD-L1 combined positive score ≥20 exhibited better ORR (80.0% vs. 28.6%, P = 0.144), median PFS (not reached vs. 7.0 months, P = 0.0137), and median OS (not reached vs. 17.8 months, P = 0.0401) compared with those with a PD-L1 combined positive score <20. Grade 3/4 treatment-related adverse events (AE) accounted for 30.0% (6/20) of AEs, and all were hematologic toxicities. Hypothyroidism was the most common immune-related AE (12/20, 60.0%).
Conclusions: Pembrolizumab plus nab-paclitaxel and platinum shows promising antitumor activity and manageable safety in patients with first-line R/M SNSCC.
Journal: Clinical Cancer Research (May 15 Issue)
Zanubrutinib plus Ixazomib and Dexamethasone in Newly Diagnosed Symptomatic Waldenström Macroglobulinemia: A Phase II Study
Purpose: Waldenström macroglobulinemia (WM) is a rare type of lymphoma, with no optimal treatment. Bruton’s tyrosine kinase inhibitors have shown promising outcomes, yet achieving deep remission (very good partial remission or complete remission) remains challenging. and time-limited therapy with proteasome inhibition has not been reported. We conducted a phase II clinical trial (NCT04463953) to evaluate the efficacy and safety of combining zanubrutinib, ixazomib, and dexamethasone (ZID) in patients with newly diagnosed WM.
Patients and Methods: A total of 27 patients were enrolled in the study. Patients received ZID induction therapy for up to six 28-day cycles, followed by consolidation therapy for a total of 24 cycles. The primary endpoint was the deep remission rate.
Results: Overall, 24 of the 27 enrolled patients completed induction treatment. One patient (4.2%) achieved complete remission. Ten patients (41.6%) achieved very good partial remission. The overall, major, and deep remission rates were 100%, 95.8%, and 45.8%, respectively. The median time to response was 2 months (range, 1–5). Five of the 22 patients had a CXCR4 mutation, with no disparity in deep remission between the patients with and without a CXCR4 mutation (40% vs. 50%; P = 0.594). The median abnormal lymphocyte (7.6% vs. 1.6%; P = 0.0019) and plasma cells (0.28%–0.02%; P = 0.0306) in bone marrow were significantly reduced after treatment. The median follow-up was 30.9 months (range, 15–42). The estimated median progression-free survival and overall survival were 40 months (95% confidence interval, 35.5–44.5) and not reached, respectively, with no difference in patients with/without CXCR4 mutations. The most common adverse event was hematologic toxicity.
Conclusions: The ZID regimen might offer deep remission and provide a time-limited Bruton’s tyrosine kinase inhibitor therapy in patients with WM.
Journal: Molecular Cancer Research
PAX8 Interacts with the SWI/SNF Complex at Enhancers to Drive Proliferation in Ovarian Cancer
Activation of lineage-specific gene expression programs is mediated by the recruitment of lineage-specific transcription factors and their coactivators to chromatin. The lineage factor PAX8 drives essential gene expression in ovarian cancer cells and is required for tumor proliferation. However, the molecular details surrounding cofactor recruitment and specific activation of transcription by PAX8 remain unknown. Here, we identify an important functional interaction between PAX8 and the switch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex. We show that PAX8 can recruit SWI/SNF complexes to DNA, in which they function to open chromatin and facilitate the expression of PAX8 target genes. Genetic deletion of PAX8 results in loss of SWI/SNF from PAX8-bound enhancers, loss of expression of associated target genes, and reduced proliferation. These results can be phenocopied by pharmacological inhibition of SWI/SNF ATPase activity. These data indicate that PAX8 mediates the expression of an essential ovarian cancer proliferative program in part by the recruitment of the SWI/SNF complex, highlighting a novel vulnerability in PAX8-dependent ovarian cancer.
Implications: PAX8 recruits SWI/SNF complexes to enhancers to mediate the expression of genes essential for ovarian cancer proliferation.
This article was highlighted in the May issue.
Journal: Molecular Cancer Therapeutics
Co-blocking TIGIT and PVRIG Using a Novel Bispecific Antibody Enhances Antitumor Immunity
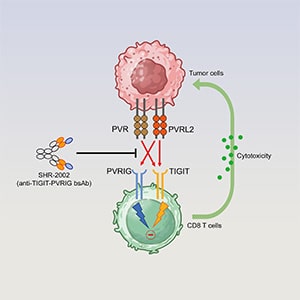
T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) and poliovirus receptor–related immunoglobulin domain (PVRIG) are immune checkpoints co-expressed on activated T and NK cells, contributing to tumor immune evasion. Simultaneous blockade of these pathways may enhance therapeutic efficacy, positioning them as promising dual targets for cancer immunotherapy. This study aimed to develop a bispecific antibody (BsAb) to co-target TIGIT and PVRIG. Expression of TIGIT and PVRIG was assessed on tumor-infiltrating lymphocytes from patients with various cancers, including non–small cell lung cancer (n = 63) and colorectal cancer (n = 26). The BsAb was engineered by fusing anti-PVRIG nanobodies to the N terminus of anti-TIGIT antibodies. Functional characterization of the BsAb was performed in vitro and in vivo, including assessments of T- and NK-cell activation and cytotoxicity. Pharmacokinetics and safety profiles were evaluated in cynomolgus monkeys. Statistical analyses were conducted using the Student t test. The results showed that the BsAb effectively blocked TIGIT and PVRIG from binding their respective ligands, CD155 and CD112, leading to significant increases in T-cell activation (2.8-fold; P < 0.05) and NK-cell cytotoxicity (1.8-fold; P < 0.05). In vivo, the BsAb demonstrated potent antitumor activity, both as a monotherapy and in combination with anti–PD-1 or anti–PD-L1, in humanized peripheral blood mononuclear cell–reconstituted and transgenic mouse models. Pharmacokinetic studies in cynomolgus monkeys revealed a favorable profile, with no dose-limiting toxicities observed after four repeated doses of 200 mg/kg. These findings provide compelling preclinical evidence for the therapeutic potential of targeting the TIGIT–PVRIG axis with a BsAb. This approach shows promise for enhancing antitumor immunity and warrants further investigation in clinical trials.
This article was highlighted and featured on the cover of the May issue.
Journal: Cancer Research Communications
Inferring Drug–Gene Relationships in Cancer Using Literature-Augmented Large Language Models
Understanding drug–gene relationships is essential for advancing targeted cancer therapies and drug repurposing strategies. However, the vast volume of biomedical literature poses significant challenges in efficiently extracting relevant insights. In this study, we developed an automated pipeline that leverages retrieval-augmented large language models (LLM) to infer drug–gene interactions using the most up-to-date biomedical literature. By integrating PubMed and state-of-the-art LLMs, our pipeline generates accurate, evidence-based inferences while addressing the limitations of static LLMs, such as outdated knowledge and the risk of producing misleading results. We systematically validated the pipeline’s performance using curated databases and demonstrated its ability to accurately identify both well-established and emerging drug targets. Using our pipeline, we constructed a pan-cancer drug–gene interaction network among hundreds of FDA-approved drugs and key oncogenes. In a case study on liver cancer, we identified and validated an association between CTNNB1 mutations and enhanced sensitivity to sorafenib, highlighting a potential therapeutic strategy for this challenging mutation. To facilitate broad accessibility, we developed GeneRxGPT, a user-friendly web application that enables cancer researchers to utilize the pipeline without programming expertise or extensive computational resources. It provides intuitive modules for drug–gene inference and network visualization, streamlining the exploration and interpretation of drug–gene relationships. We anticipate that GeneRxGPT will empower researchers to accelerate drug discovery and development, making it a valuable resource for the cancer research community.
Significance: This study presents a novel approach that integrates LLMs with real-time biomedical literature to uncover drug–gene relationships, transforming how cancer researchers identify therapeutic targets, repurpose drugs, and interpret complex molecular interactions. GeneRxGPT, our user-friendly tool, enables researchers to leverage this approach without requiring computational expertise.


