Summer is heating up, so why not keep cool by reading about some of the hottest topics in cancer research? This month’s Editors’ Picks from the 10 peer-reviewed journals of the American Association for Cancer Research (AACR) include studies on pancreatic cancer interception, breast cancer risk prediction, the tumor microenvironment, and more.
Read the abstracts of the selected articles below, and follow the links to access the full-text articles, freely available for a limited time.
Journal: Blood Cancer Discovery
A Model of Intratumor and Interpatient Heterogeneity Explains Clinical Trials of Curative Combination Therapy for Lymphoma
Models of tumor drug response have illuminated important concepts in oncology, but there remains a need for theory that combines intratumor and interpatient heterogeneity to explain patient outcomes, especially for curative treatments. In this study, we present a mathematical model of multidrug therapy that describes both cell-to-cell and patient-to-patient heterogeneity as distributions of drug sensitivity and apply it to simulate curative combination therapies for diffuse large B-cell lymphoma (DLBCL). Simulated trials reproduced progression-free survival and changes in ctDNA observed under standard therapy and accurately predicted success or failure of nine randomized trials of first-line combinations based on drug efficacies in relapsed/refractory DLBCL. Finally, we used the model to explore how drug synergies, biomarkers, and subtype-specific endpoints could improve the chance of success of targeted combination therapies. This study offers a quantitative model of curative drug combinations and suggests that predictive simulations could aid the design of new regimens with curative intent.
Significance: A new model of intratumor and interpatient heterogeneity in response to drug combinations explains and predicts the results of clinical trials of curative-intent treatments for DLBCL. This model can be used to understand and inform optimal design of curative drug combinations and clinical trials.
A related commentary was published in the May issue.
Journal: Cancer Discovery
Long-Term Latency of Highly Mutated Cells in Normal Mouse Skin Is Reversed by Exposure to Tumor Promoters and Chronic Tissue Damage
Historical studies performed nearly a century ago using mouse skin models identified two key steps in cancer evolution: initiation, a likely mutational event, and promotion, driven by inflammation and cell proliferation. Initiation was proposed to be permanent, with promotion as the critical rate-limiting step for cancer development. In this study, we carried out whole-genome sequencing to demonstrate that initiated cells with thousands of mutagen-induced mutations can persist for long periods and are not removed by cell competition or by immune intervention, thus mimicking the persistence of cells with cancer driver mutations in normal human tissues.
In the mouse, these cells do not give rise to tumors unless exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). Tissue damage and regenerative proliferation, but not normal cell turnover, consistently trigger tumor formation. Wounding, promoter treatment, and obesity enhance promotion without increasing mutational burden, supporting the possibility of future cancer prevention efforts directed at promotional risk factors.
Significance: Using historical skin cancer models, we reveal that mutated cells can persist without tumor formation and give rise to cancer upon exposure to tumor promoters, underscoring the importance of tumor promotion over initiation as the rate-limiting step in carcinogenesis and the need for cancer prevention strategies targeting promotional factors.
This article was featured on the cover of the June issue, which also included a related commentary.
Journal: Cancer Epidemiology, Biomarkers & Prevention
The Breast Tumor Immune Microenvironment of DNA Double-Strand Break Repair Pathogenic Variant Carriers Is Enriched with Tumor-Associated Macrophages
Background: Approximately 5% of patients with breast cancer have a rare pathogenic germline genetic variant that is associated with increased breast cancer risk. Mutations in more than 12 genes have been associated with hereditary breast cancer risk, many of which are involved in genome stability pathways, including DNA double-strand break (DSB) repair. We hypothesized that carriers of DSB repair–related pathogenic variants (PV) may have a distinct tumor immune environment that differs from that of noncarriers.
Methods: We utilized tumor transcriptome data from 559 participants with invasive breast cancer from the Nurses’ Health Studies and Nurses’ Health Studies II to infer immune-related gene expression signatures and immune cell abundance.
Results: Thirty-three (5.9%) individuals had germline DSB repair–related PVs in one or more of the following genes: ATM, BARD1, BLM, BRCA1, BRCA2, BRIP1, CHEK2, FANCC, FANCM, NBN, PALB2, RAD50, RAD51C, and/or RECQL. In covariate-adjusted analyses, DSB repair–related PV carrier status was positively associated with both a STAT1 signature (standardized β = 0.59; P = 3.5 × 10−3) and inferred M1 macrophage infiltration (standardized β = 0.56; P = 1.4 × 10−3). Furthermore, these immune features correlated with other features related to tumor IFN response signaling, suggesting that this enrichment is occurring in an inflammatory context.
Conclusions: These results indicate that breast tumors of DSB repair–related PV carriers have distinct immune features, which may have therapeutic implications in this high-risk population.
Impact: These results support further characterization of macrophage characteristics and abundance in the breast tumor microenvironment of DSB repair–related PV carriers.
Journal: Cancer Immunology Research
Mesenchymal Stem Cells and Fibroblasts Contribute to Microvascular Proliferation in Glioblastoma and are Correlated with Immunosuppression and Poor Outcome
Microvascular proliferation (MVP) is a disease-defining hallmark of glioblastoma and other World Health Organization grade 4 gliomas. MVP also serves as a poor prognostic marker in various solid tumors. Despite its clinical significance, the mechanisms and biological consequences of MVP are controversial and remain unclear. In this study, we performed single-cell RNA sequencing on paired CD45−CD105+ vascular/perivascular stromal cells (PVSC) and CD45+CD105± immune cells from 16 primary glioma patient samples, both with and without MVP. This analysis revealed the presence of developmentally related mesenchymal stem cells alongside cancer-associated fibroblasts, pericytes, fibromyocytes, and smooth muscle cells within the CD45−CD105+ compartment. RNA velocity analysis identified PDGFRB as a putative driver gene guiding mesenchymal stem cells toward more mature PVSCs in the context of MVP. Signaling network analysis and digital spatial profiling uncovered interactions between PDGFRB+ PVSCs and immunosuppressive myeloid cell subsets enriched in the perivascular niche, suggesting targetable receptor–ligand interactions. Additionally, a gene signature of MVP-associated PVSCs from gliomas predicted worse prognosis in multiple other solid tumors. This study provides a transcriptomic cell atlas of PVSCs and immune cells in glioma, helping to refine the biological model of MVP which has traditionally focused on endothelial cells.
This article was featured on the cover of the June issue.
Journal: Cancer Prevention Research
Deriving a Mammogram-Based Risk Score from Screening Digital Breast Tomosynthesis for 5-Year Breast Cancer Risk Prediction
Screening digital breast tomosynthesis (DBT) aims to identify breast cancer early when treatment is most effective, leading to reduced mortality. In addition to early detection, the information contained within DBT images may also inform subsequent risk stratification and guide risk-reducing management. Using transfer learning, we refined a model in the Joanne Knight Breast Health Cohort at Washington University, a cohort of 5,066 women with DBT screening (mean age, 54.6), among whom 105 were diagnosed with breast cancer (26 ductal carcinoma in situ). We applied the model to external data from the Emory Breast Imaging Dataset, a cohort of 7,017 women free from cancer (mean age, 55.4), among whom 111 pathology-confirmed breast cancer cases were diagnosed more than 6 months after initial DBT (17 ductal carcinoma in situ). We obtained a 5-year AUC of 0.75 [95% confidence interval (CI), 0.73–0.78] in the internal validation. The model validated in external data gave an AUC of 0.72 (95% CI, 0.69–0.75). The AUC was unchanged when age and Breast Imaging-Reporting and Data System density were added to the model with synthetic DBT images. The model significantly outperforms the Tyrer-Cuzick model, with a 5-year AUC of 0.56 (95% CI, 0.54–0.58; P < 0.01). Our model extends risk prediction applications to synthetic DBT, provides 5-year risk estimates, and is readily calibrated to national risk strata for clinical translation and guideline-driven risk management. The model could be implemented within any digital mammography program.
Prevention Relevance: We develop and externally validate a 5-year risk prediction model for breast cancer using synthetic DBT and demonstrate clinical utility by calibrating to the national risk strata as defined in breast cancer risk management guidelines.
Journal: Cancer Research (June 1 issue)
FGFR2 Abrogation Intercepts Pancreatic Ductal Adenocarcinoma Development

Activating KRAS mutations are a key feature of pancreatic ductal adenocarcinoma (PDAC) and drive tumor initiation and progression. However, mutant KRAS by itself is weakly oncogenic. Defining the pathways that cooperate with mutant KRAS to induce tumorigenesis could identify prevention and treatment strategies. Analyzing organoids and murine and human pancreatic specimens, we found that the receptor tyrosine kinase FGFR2 was progressively upregulated in mutant KRAS-driven metaplasia, precancerous lesions, and classical PDAC. In genetic mouse models, FGFR2 inactivation impeded mutant KRAS-driven transformation of acinar cells by reducing proliferation and MAPK pathway activation. FGFR2 abrogation significantly delayed tumor formation and extended the survival of these mice. Furthermore, FGFR2 collaborated with EGFR, and dual blockade of these receptor signaling pathways significantly reduced mutant KRAS-induced precancerous lesion formation. Together, these data have uncovered a pivotal role for FGFR2 in the early phases of pancreatic tumorigenesis, paving the way for future therapeutic applications of FGFR2 inhibitors for pancreatic cancer interception.
Significance: FGFR2 inhibition reduces mutant KRAS signaling, which can impair mutant KRAS-expressing pancreatic cancer precursor lesions that are prevalent in the average healthy adult and delay pancreatic ductal adenocarcinoma progression.
This article was featured on the cover of the June issue and highlighted in an AACR press release.
Journal: Cancer Research (June 15 issue)
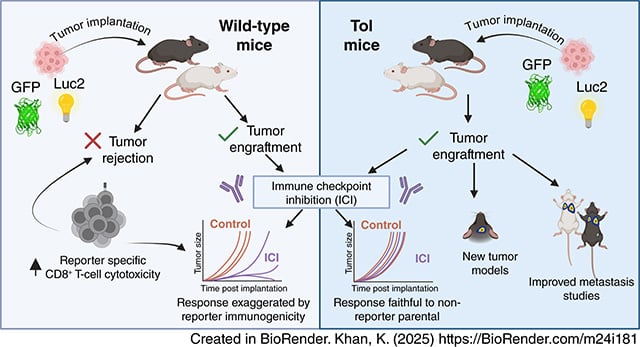
Immunological Tolerance to Luciferase and Fluorescent Proteins Using Tol Mice Enables Development of Improved Tumor Models for Investigating Immunity and Metastasis
There is a continuing need for improved preclinical mouse models of cancer that more accurately predict therapy outcomes for future clinical translation. Luciferase and bioluminescence have long been utilized to generate models conducive to noninvasive imaging to monitor tumor growth, disease progression, and response to therapy. However, luciferase, as well as fluorescent reporter proteins, are highly immunogenic, limiting their use in some syngeneic tumor models in immunocompetent mice. In this study, we described the utility of transgenic mice engineered to have tolerance to luciferase and several other reporter proteins, known as Tol mice, in cancer immunology research. Some tumor cell lines expressing both luciferase and GFP were completely rejected in wild-type mice but maintained robust growth in Tol mice. Additionally, Tol mice allowed the development of an experimental brain metastasis model and a postsurgical resection spontaneous metastasis model. Importantly, even when certain cell lines carrying reporter proteins successfully formed tumors in immunocompetent wild-type mice, underlying immunity existed that could be reinvigorated by immune checkpoint inhibitors. Therefore, caution is needed when using such models in wild-type mice, as exaggerated effects may be induced by immunotherapy. Tol mice circumvent this problem and will likely widen the number of orthotopic and metastatic tumor models that can be used in immunotherapy studies in both C57Bl/6 and BALB/c mice.
Significance: Tol transgenic mice, tolerant to reporter proteins like luciferase and GFP, can be used to develop improved tumor models for studying metastasis and immunotherapy, avoiding immune rejection issues in immunocompetent mice.
A related commentary was published in the June 15 issue.
Journal: Clinical Cancer Research (June 1 issue)
A Phase II Study of Fulvestrant plus Abemaciclib in Hormone Receptor–Positive Advanced or Recurrent Endometrial Cancer
Purpose: Inhibition of the cyclin D–cyclin-dependent kinase (CDK)4/6–INK4–retinoblastoma pathway can overcome acquired or de novo treatment resistance to endocrine monotherapy. Responses to endocrine monotherapy in advanced endometrial cancer are suboptimal, perhaps due to genomic alterations that promote estrogen receptor–independent cyclin D1–CDK4/6 activation. We hypothesized that the addition of abemaciclib, a CDK4/6 kinase inhibitor, to antiestrogen therapy with fulvestrant would be an effective therapeutic strategy in patients with advanced or recurrent endometrial cancer.
Patients and Methods: In this phase II study, patients with advanced or recurrent endometrial cancer received 150 mg of abemaciclib orally twice daily with 500 mg of fulvestrant intramuscularly monthly with a 2-week loading dose. Eligibility included estrogen receptor or progesterone receptor expression ≥1% by IHC, measurable disease, ≤2 prior lines of chemotherapy, and ≤1 prior lines of hormonal therapy. The primary endpoint was the objective response rate by RECIST v1.1.
Results: Twenty-seven patients initiated therapy, and 25 were evaluable for efficacy. Eleven patients achieved partial response; 10 responses (91%) were in copy number–low/no specific molecular profile tumors, 1 response (9%) was in a microsatellite instability–high tumor, and no responses were observed in copy number–high/TP53 abnormal tumors. The objective response rate was 44% (90% confidence interval, 27.0%–62.1%). The median duration of response was 15.6 months. The median progression-free survival was 9.0 months (90% confidence interval, 1.8–20.4). The most common grade ≥3 treatment-related adverse events were neutropenia (26%) and anemia (19%); no new safety signals were identified.
Conclusions: The combination of abemaciclib and fulvestrant has promising activity with durable responses in advanced or recurrent endometrial cancer; a randomized trial is planned.
A related commentary was published in the June 1 issue.
Journal: Clinical Cancer Research (June 15 issue)
Randomized Phase III Study of EGFR Tyrosine Kinase Inhibitor and Intercalated Platinum-Doublet Chemotherapy for Non–Small Cell Lung Cancer Harboring EGFR Mutation
Purpose: This study was performed to confirm the superiority in overall survival (OS) of EGFR tyrosine kinase inhibitor (TKI gefitinib or osimertinib) monotherapy versus EGFR TKI with intercalation of cisplatin plus pemetrexed as the first-line treatment for patients with advanced non-squamous non–small cell lung cancer (NSqNSCLC) harboring EGFR mutation.
Patients and Methods: This was an open-label, multicenter, randomized phase III study. Patients with chemotherapy-naïve advanced or recurrent NSqNSCLC harboring EGFR mutation (exon 19 deletion or exon 21 L858R point mutation) were randomly assigned (1:1) to EGFR-TKI monotherapy or the EGFR TKI plus intercalated chemotherapy group. The primary endpoint was OS, and the secondary endpoints included progression-free survival (PFS).
Results: From December 2015 to October 2020, 501 patients were randomized. The EGFR TKI was changed from gefitinib to osimertinib in October 2018 (gefitinib cohort: n = 308 and osimertinib cohort: n = 193). There was no survival advantage in the EGFR TKI plus intercalated chemotherapy group; the median survival time of both groups was 48.0 months (HR, 0.985; 91.4% confidence interval, 0.796–1.219; one-sided P = 0.4496). The median PFS time was 12.0 months in the EGFR-TKI monotherapy group and 18.0 months in the EGFR TKI plus intercalated chemotherapy group (HR, 0.762; 95% confidence interval, 0.628–0.925; one-sided P = 0.003). The OS and PFS trends in both gefitinib and osimertinib cohorts were identical to those in the entire population.
Conclusions: The intercalation of cisplatin plus pemetrexed after the response to EGFR TKI improved PFS but not OS compared with EGFR TKI monotherapy as the first-line treatment for patients with advanced NSqNSCLC harboring EGFR mutation.
Journal: Molecular Cancer Research
SIRT2 Regulates the SMARCB1 Loss-Driven Differentiation Block in ATRT
An atypical teratoid rhabdoid tumor (ATRT) is a highly aggressive pediatric brain tumor driven by the loss of SMARCB1, which results in epigenetic dysregulation of the genome. SMARCB1 loss affects lineage commitment and differentiation by controlling gene expression. We hypothesized that additional epigenetic factors cooperate with SMARCB1 loss to control cell self-renewal and drive ATRT. We performed an unbiased epigenome-targeted screen to identify genes that cooperate with SMARCB1 and identified SIRT2 as a key regulator. Using in vitro pluripotency assays combined with in vivo single-cell RNA transcriptomics, we examined the impact of SIRT2 on differentiation of ATRT cells. We used a series of orthotopic murine models treated with SIRT2 inhibitors to examine the impact on survival and clinical applicability. We found that ATRT cells are highly dependent on SIRT2 for survival. Genetic or chemical inhibition led to decreased cell self-renewal and induction of differentiation in tumor spheres and in vivo models. We found that SIRT2 inhibition can restore gene expression programs lost because of SMARCB1 loss and reverse the differentiation block in ATRT in vivo. Finally, we showed the in vivo efficacy of a clinically relevant inhibitor demonstrating SIRT2 inhibition as a potential therapeutic strategy. We concluded that SIRT2 is a critical dependency in SMARCB1-deficient ATRT cells and acts by controlling the pluripotency–differentiation switch. Thus, SIRT2 inhibition is a promising therapeutic approach that warrants further investigation and clinical development.
Implications: SIRT2 inhibition is a molecular vulnerability in SMARCB1-deleted tumors.
Journal: Molecular Cancer Therapeutics
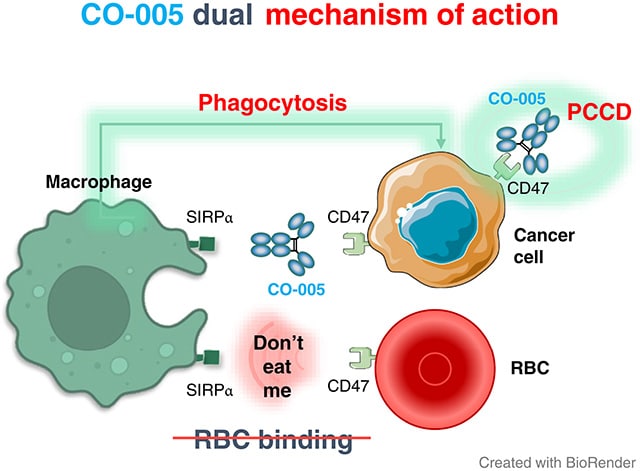
Development of a Novel Bifunctional Anti-CD47 Fusion Protein with Improved Efficacy and a Favorable Safety Profile
Therapeutic anti-CD47 monoclonal antibodies (mAbs) are designed to block the CD47–SIRPα checkpoint and promote immune-mediated recognition and elimination of cancer cells. However, current anti-CD47 mAbs have limitations, including off-tumor toxicity and reduced effectiveness in advanced cancers. Additionally, CD47 serves as a death receptor that mediates programmed cancer cell death (PCCD), a mechanism that has not been fully explored in current therapies. In this study, we introduce CO-001, a chimeric bifunctional IgG4 mAb, and its optimized variant CO-005, a bivalent humanized single-chain fragment variable–fragment crystallizable fusion protein. Both CO-001 and CO-005 promoted phagocytosis and PCCD. CO-005, specifically engineered to overcome the safety limitations associated with anti-CD47 antibodies, demonstrates a superior hematologic safety profile in vitro and ex vivo compared with benchmark anti-CD47 antibodies. Notably, CO-005 exhibited no binding to red blood cells, limited binding to white blood cells, and showed no hemagglutination activity. In preclinical models, CO-005 demonstrated potent antitumor activity in B-cell precursor acute lymphoblastic leukemia and Raji lymphoma xenograft models through the dual action of PCCD induction and enhancement of phagocytosis. The ability of CO-005 to trigger strong PCCD while preserving conventional immune responses provides a novel and promising approach for CD47-targeted cancer therapy. Its favorable safety profile, observed in both in vitro and ex vivo studies, positions CO-005 as a promising candidate with potential therapeutic advantages over existing anti-CD47 treatments.
Journal: Cancer Research Communications
Breast Cancer Risk Modification in Women with Pathogenic Variants in BRCA1, BRCA2, ATM, CHEK2, and PALB2
There are limited prospective data on whether established risk factors modify breast cancer risk in women with pathogenic variants (PV) in BRCA1/2 and virtually no risk modification data for ATM, CHEK2, or PALB2. We conducted a nested case–control study in the Women’s Health Initiative (WHI), randomly selecting women with and without breast cancer for DNA sequencing. We evaluated breast cancer odds associated with obesity, family cancer history, smoking, alcohol, parity, breastfeeding, oophorectomy, tubal ligation, neighborhood socioeconomic status, and menopausal hormone therapy (MHT) with estrogen and progestin or estrogen only in PV carriers and noncarriers. In exploratory analyses, we grouped genes by established predisposition for estrogen receptor (ER)-positive (ATM and CHEK2) or ER-negative (BRCA1 and PALB2) disease. Multivariable models with interaction terms were used to assess differential risk modification by PVs. Among 12,957 WHI participants, 287 carried PVs. Breastfeeding was modestly associated with reduced risk for PALB2 [OR = 0.08; 95% confidence interval (CI), 0.00–0.92; P value = 0.042]. With one-sided 95% CI, power was sufficient to exclude OR ≥2.0 with obesity for ATM and BRCA2; smoking and alcohol for CHEK2; no breastfeeding for ATM; no oophorectomy for BRCA2 and CHEK2; no tubal ligation for CHEK2; and neighborhood socioeconomic status for all genes. Estrogen + progestin MHT was modestly associated with increased risk for ER-positive PVs (OR = 7.31; 95% CI, 1.14–64.20; P = 0.036). PVs did not modify risk (interaction P ≥ 0.05). BRCA1/2, ATM, CHEK2, and PALB2 PV carriers do not have breast cancer OR ≥2.0 with many established risk factors. However, MHT warrants additional study in PV carriers.
Significance: There is limited information on whether established risk factors increase breast cancer risk from PVs. In the WHI, PV carriers had no substantial (≥2-fold) increase with most risk factors, except potentially MHT in ATM or CHEK2 carriers. The results may inform counseling and research on MHT.



