For patients and doctors facing acute myeloid leukemia (AML), one of the biggest challenges is the disease’s complexity, owing to the many forms it assumes. Even within an individual patient, AML is often a heterogeneous collection of different cell types and states, which makes it difficult to predict how the overall disease will progress or respond to treatment.
Fortunately, a new study unveiled at the AACR Annual Meeting 2025—and simultaneously published in the AACR journal Blood Cancer Discovery along with a commentary—characterized the diverse spectrum of AML and provided clarity, plus new research tools, that could fuel important progress in the field.
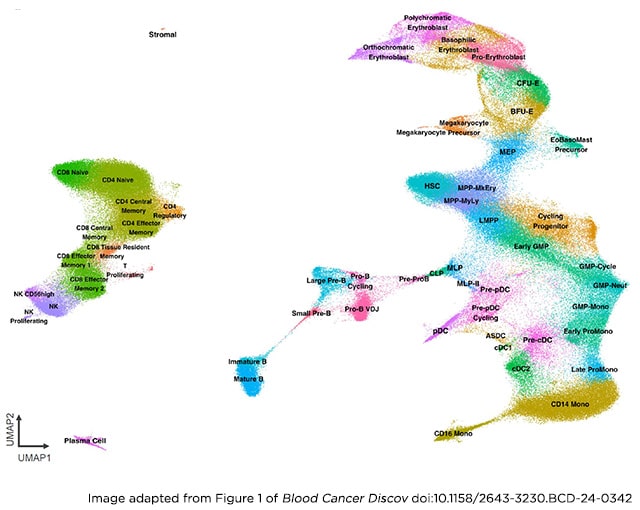
By combining cutting-edge sequencing with a vast collection of patient samples, a team led by John E. Dick, PhD, FAACR, a senior scientist at the Princess Margaret Cancer Centre and a member of the AACR Hematologic Malignancies Working Group Steering Committee, created an atlas of normal blood cell development and then used this reference map to decipher 12 distinct cell-state patterns observed in human AML.
“We wanted to link this cell-state heterogeneity to the genetic drivers that initiate the disease and to the varying clinical outcomes of these patients,” said first author Andy Zeng, an MD/PhD student at the University of Toronto who presented the new research during the “Cutting-Edge Advances in Hematology” Minisymposium at the AACR Annual Meeting 2025.
Building BoneMarrowMap, an Atlas of Blood Cell Development
The first step was to create the reference atlas—called BoneMarrowMap—and establish a baseline for normal hematopoiesis, the process of blood cell development that gives rise to the body’s many specialized blood and immune cells.
“It’s important to study normal hematopoiesis because we’re trying to understand how differentiation goes wrong in leukemia. To do that in a precise way, we first need to understand the parameters of normal blood development and clearly define the specific cell states that exist across the hematopoietic hierarchy,” Zeng explained.
To do so, Dick, Zeng, and their colleagues leveraged single-cell RNA sequencing capable of tracking the expression of thousands of genes in each cell simultaneously to define cell states with consistency.
Overall, BoneMarrowMap helped define more than 50 normal blood and immune cell states capturing both early stem and progenitor populations as well as mature cell types that have differentiated along diverging lineages.
Revealing a Dozen Different Acute Myeloid Leukemias
Equipped with a comprehensive reference to compare leukemia against, the team then mapped 1.2 million leukemia cells from more than 300 patients with AML onto BoneMarrowMap. They uncovered, as Zeng described, “a diverse landscape of aberrant differentiation” and identified 12 recurring patterns that reflect different ways normal blood cell development can get derailed and send cells down the track toward leukemia.
Some leukemias seemed stuck at an early stem cell-like stage; others were skewed toward more mature lineages. A few patterns were linked to unexpected identities, such as lymphoid (white blood cell)-like or erythroid (red blood cell)-like states, blurring the line between AML and rare leukemias.
Because each pattern links to distinct biology, this classification helps explain why two patients with a diagnosis of AML can experience drastic differences in terms of disease progression and treatment response. Patients whose disease was dominated by early lymphoid subsets, for example, were more likely to experience worse survival, whereas patients whose disease was abundant in early erythroid subsets tended to fare better.
To probe what genetic alterations drive each pattern, the researchers paired their cell-state map with mutational data from 1,200 additional patients with AML. While many common driver mutations were associated with particular cell states, considerable heterogeneity was observed, with no clear-cut relationship between specific genetic drivers and cell states. Essentially, the same mutation can drive disease progression down different trajectories based on the identity of the cell it occurs in and the other mutations the cell carries.

“Our findings extend beyond genetics alone. Cellular context mattered, too,” said Zeng, regarding the rules that govern AML evolution.
In his view, the work also illuminates “the murky diagnostic boundaries” between some forms of AML and other leukemias that “prompt us to revisit the diagnostic distinctions that we have traditionally drawn between these disease categories.” He also stressed the need for better profiling of underrepresented granulocyte-like leukemias as well as extremely rare—sometimes one in a million, according to Zeng—leukemia stem cells that can exert significant impact on the course of AML.
Crucially, the atlas also hints at how treatment might be tailored to specific AML subtypes. When the researchers revisited laboratory drug-response data, they saw clear contrasts between two of the newly defined patterns. For example, compared with leukemias dominated by an erythroid-like program, leukemias dominated by a lymphoid-like program were more sensitive to tyrosine kinase inhibitors. Although data on the other patterns are still emerging, these early signals suggest that some AML subtypes carry unique therapeutic vulnerabilities—knowledge that could help match patients to drugs likely to be effective against their particular disease and shape future clinical trial designs around those differences.
Why a Hematopoietic Atlas Matters for Acute Myeloid Leukemia Patients
This study’s potential impact lies in advances that begin with AML diagnosis and flow through to therapy and future research. By exposing which developmental program dominates an individual’s leukemia, the atlas may eventually be able to help clinicians refine prognostic outlooks in a timely fashion, sorting aggressive, treatment-resistant cases from more indolent ones even when their genetic blueprints look alike.
On the treatment front, insights like the lymphoid- versus erythroid-specific drug sensitivities could translate into more personalized care. By linking a patient’s leukemia subtype to therapies shown to work best against that pattern, doctors can potentially steer patients away from ineffective regimens and toward drugs with a higher chance of success. Likewise, clinical trials can be structured around the atlas’s classification, enrolling patients whose leukemia biology matches the drug’s target and speeding the path toward precision treatment for AML.
Underpinning these potential clinical benefits is BoneMarrowMap itself, a freely available software package that scientists can use to improve our understanding of the various forms of AML, as well as identify new targets to develop treatments against.
By marrying large-scale single-cell sequencing data with genetic analysis and patient outcomes, this exciting work turned AML’s notorious variability from a black box into a navigable landscape. And Zeng believes this same framework—comparing malignant cells with a high-resolution reference of their healthy counterparts—can be applied to other types of cancer, too: “We hope this motivates others outside of the AML space to adopt similar analytical frameworks that allow them to integrate genetic and cellular models of cancer heterogeneity in order to advance precision medicine across oncology.”



